Fats – The Good, The Bad and The Essential
/Fats have long been seen as a bad four-letter word. It’s something we are told to avoid, and low-fat diets have been encouraged for years with the promise of keeping us healthy. However, having some fats in our diet is essential for our bodies to function.
Why are fats so important?
Fats are the main storage form of energy in our bodies; there is more than twice as much energy stored in a gram of fat compared to a gram of sugar.
But fats are not just a great source of energy. They are important to help the body absorb the fat-soluble vitamins A, D, E and K. Fats are an essential part of our cells; found in cell membranes and sheaths of nerve cells. And many pathways, such as those that control growth, immunity, and reproduction, require fats to act as messengers in chemical reactions.
Extra fat that we consume is stored in special fat cells called adipose cells. These fatty stores also help to insulate our bodies and cushion our organs. Storing too much fat, however, can lead to serious health concerns such as cardiovascular disease, obesity and diabetes.
What are fats?
Fats are lipids, and lipids are a diverse group of molecules that share a common trait – they are all hydrophobic. This simply means that they don’t mix with water (hydro = water, phobic = fear).
Lipids vary in structure and function, but for now let’s just consider the neutral fats triglycerides, as they are how our bodies store fats for energy.
What are triglycerides?
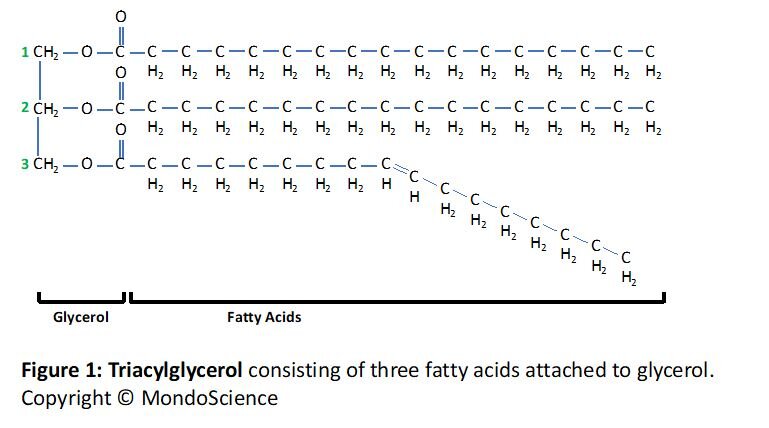
Also known as triacylglycerols or simply fats, triglycerides are common large lipids that are made up of three (tri) fatty acid chains linked to glycerol, as illustrated in Figure 1. Each fatty acid consists of a hydrocarbon chain (made up of hydrogen and carbon) which gives the molecule its hydrophobic property.
What are saturated fats and unsaturated fats and their differences?
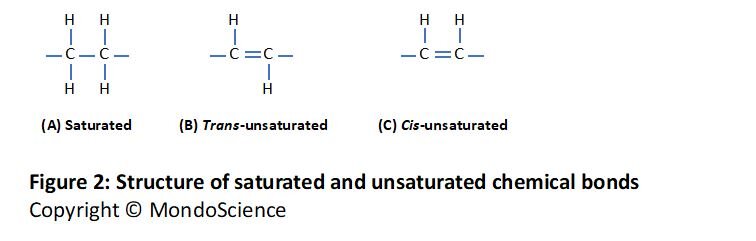
Fatty acids with one or more double bonds are called unsaturated fatty acids, while fatty acids with no double bonds are called saturated fatty acids. They are called saturated fats because the hydrocarbon chain has the maximum number of hydrogen atoms, i.e. they are ‘saturated’ with hydrogen. An unsaturated fatty acid has one less hydrogen atom on each carbon of a double bond (see Figure 1 above and 2 below).
An unsaturated fatty acid with one double bond is known as mono-unsaturated (mono means one), while those with two or more double bonds are called poly-unsaturated (poly means many).
Some unsaturated fatty acid chains are straight while others have kinks in them. The double bonds that cause the kinks are called cis-double bonds (Figure 3C). Other double bonds, called trans-double bonds, don’t cause a kink and these unsaturated fatty acids are straight (Figure 3B) with a structure similar to saturated fatty acids (Figure 3A). Nearly all naturally occurring unsaturated fatty acids have cis-double bonds. Figure 2 illustrates the structure of these bonds and the position of the hydrogen atoms on the carbons involved in the double bonds.
Properties of Fats
The physical properties of fats mainly depend on the length of the hydrocarbon chain and its degree of unsaturation (i.e. how many double bonds it has).
The longer the fatty acid chain is, the less soluble (more hydrophobic) it becomes in water. Remember the hydrocarbon chain is what gives it it’s hydrophobic property.
Saturated fatty acids are extremely flexible due to the free rotation around each of the carbon-carbon bonds; there are no double bonds to hinder its movement. These saturated fatty acids can also pack very tightly together because of their straight structure, making fats that contain them solid at room temperature. On the other hand, the kinks in unsaturated fatty acids prevent them from packing tightly together, and so fats containing these are liquid at room temperature. The more double bonds the more liquid they are. Keeping in mind that trans-unsaturated fatty acids have a similar structure to saturated fats, do you think Trans fats are solid or liquid at room temperature? (Answer below).
What is the main function of fats/ lipids?
The main function of fats is long-term energy storage. As already mentioned, there is twice as much energy stored in a gram of fat than in a gram of sugar. And because they can pack tightly together, fats are less bulky and easier for us to carry around than larger sugar stores. It is worth noting that humans can only store a day’s supply of sugar in the body but can store enough fat to survive for months.
In addition to storing fats, fatty tissue (adipose tissue) helps to cushion vital organs and insulate the body.
Other major lipids with essential functions include:
Phospholipids – the main structural component of every cell membrane
Steroid hormones – biological signals that activate the expression of specific genes
Cholesterol – used to make steroid hormones, including sex hormones, and found in cell membranes
Bile acids – derivatives of cholesterol that help to emulsify fats in the intestine to aid their digestion
Fat soluble vitamins A, D, E and K – obtained mainly in our diet and required for vision, calcium absorption, antioxidant to prevent cell damage, and blood clotting respectively
Lipoproteins – required for transport of fats and cholesterol; these include HDL (good) and LDL (bad)
Good Fats vs Bad Fats
The terms saturated fats and unsaturated fats are often used when talking about fats in our diet. You should now be getting an idea of which fats are good fats and which are the bad fats we need to avoid.
Saturated fats are bad fats because they are made up of saturated fatty acids. Remember this type of fat has a straight structure (see Figure 2A) so they can pack tightly together. Because of this, saturated fats such as butter are solid at room temperature. A diet rich in saturated fats contributes to cardiovascular disease (atherosclerosis). This is because large amounts of these fats form deposits called plaques that build up in blood vessels, thus reducing the flow of blood and weakening the vessel. Consuming high amounts of saturated fats will also contribute to weight gain, insulin resistance and Diabetes Mellitus Type 2.
In contrast, unsaturated fats, the good fats, cannot pack as tightly together due to the kink in their structure (see Figure 2C).
Most natural fats found in vegetable oils and animal fat will have a mixture of saturated and unsaturated fats. Vegetable oils, for example olive and corn oils, contain more unsaturated fats making them liquid at room temperature. Fats such as butter will contain saturated fats and some unsaturated fats, so that they are solid but a bit soft. Animal fat, such as beef fat, will largely contain saturated fats and are solid at room temperature.
The Essential Fats
Our bodies can make most of the fatty acids we need, however, there are still some that we can’t make. These essential fatty acids must to be obtained from our diets. For example, linoleic acid, also known as omega-6, is an essential fatty acid needed to make the phospholipids found in cell membranes. a-Linolenic acid, also known as omega-3, is another essential fatty acid.
Fats and Nutrition
Dietary government guidelines recommend no more than 35% of our total food energy to come from fats. Of this, saturated fats should be <10% of the total, and Trans fats <1% or avoided all together (WHO recommendations). Replacing saturated fats with unsaturated fats is also encouraged.
Despite containing mainly unsaturated fats, not all vegetable oils are good for you. Manufacturers hydrogenate vegetable oils to make cooking oils, margarine, peanut butter etc. This is to give them a longer shelf-life, make them more stable at higher temperatures (cooking oils) and stop them from separating (margarine and peanut butter). The process of partially hydrogenation (adding hydrogen) converts some of the unsaturated fats to saturated fats; cis-double bonds (Figure 2C) are converted to single bonds (Figure 2A). This process will also convert 30-50% of the cis-double bonds to trans-double bonds (Figure 2B).
A high intake of Trans fats in the diet contributes to cardiovascular disease and an increase in LDL (bad cholesterol). Like saturated fats, Trans fats can pack very tightly together due to their structure. Most processed, fast and frozen foods, as well as baked products like cakes and biscuits, contain high amounts of Trans fats.
The average amount of Trans fats we consume has dropped over recent years to below the recommended maximum. This is partly due to strict government guidelines. However, you should always read the label of products as some manufactures have replaced Trans fats with saturated fats to keep the product solid, especially in the case of biscuits.
Low-fat diets have been popular for years, yet there is no scientific evidence that following these diets have any benefit with regards to your risk of cardiovascular disease. It is far better to follow a healthy eating pattern, eating a variety of nutrient rich foods across all food groups, within a healthy calorie limit (~2000kcal for a woman and ~2500kcal for a man per day), while limiting your intake of saturated fats, added sugar and sodium. The Eatwell Guide is a good guide of how much of each food group is needed for a healthy balanced diet. In addition, Table 1 (adapted from: Mozaffarain et al., 2011) lists the types of foods you should be eating more or less of for a healthier balanced diet.
In summary, we all need a bit of fat in our diet.
Eat plenty of fruit, vegetables, whole grains, lean protein and fish to maintain a healthy diet that includes the essential fatty acids. Try to limit your intake of processed and fried fast foods, which are high in saturated and Trans fats, and replace them with healthier mono- and poly-unsaturated fats.
Click here to learn more about lipids as macromolecules in the free MondoScience course.
Some other post we think you will like: ‘What Are Amino Acids’, ‘100% Amino Acid Score’, ‘Top 5 Health Benefits of Chewing Longer’.
If you enjoyed reading this, please like, share or leave a comment.